Radithor
From Wikipedia, the free encyclopedia
Radithor was a patent medicine that is a well-known example of radioactive quackery and specifically of excessively broad and pseudoscientific application of the principle ofradiation hormesis. It consisted of triple distilled water containing at a minimum 1 microcurie (37 kBq) each of the radium 226 and 228 isotopes.
History[edit]
Radithor was manufactured from 1918 to 1928 by the Bailey Radium Laboratories, Inc., of East Orange, New Jersey. The owner of the company and head of the laboratories was listed as Dr. William J. A. Bailey, a dropout from Harvard College,[1] who was not a medical doctor.[2] It was advertised as "A Cure for the Living Dead"[3] as well as "Perpetual Sunshine".
Eben Byers, a wealthy American socialite, athlete, industrialist and Yale College graduate, died from Radithor radium poisoning in 1932.[4]
The Wall Street Journal article describing the Byers incident was called "The Radium Water Worked Fine Until His Jaw Came Off".[5] Byers's death led to the strengthening of theFood and Drug Administration's powers and the demise of most radiation-based patent medicines.
Properties of water
From Wikipedia, the free encyclopedia
"H2O" and "HOH" redirect here. For other uses, see H2O (disambiguation) and HOH (disambiguation).
"Hydrogen monoxide" redirects here. For the prank involving the chemical name of water, see Dihydrogen monoxide hoax.
This article is about the physical and chemical properties of pure water. For general discussion and its distribution and importance in life, see Water. For other uses, seeWater (disambiguation).
Water (H
2O)


Names IUPAC name
water, oxidane
Other names
Hydrogen oxide, Dihydrogen monoxide(DHMO), Hydrogen monoxide, Dihydrogen oxide, Hydrogen hydroxide (HH or HOH), Hydric acid, Hydrohydroxic acid, Hydroxic acid, Hydrol,[1] μ-Oxido dihydrogen
Identifiers
7732-18-5 
ChEBI CHEBI:15377 
ChEMBL ChEMBL1098659 
ChemSpider 937 
Jmol 3D image Interactive graph PubChem 962 RTECS number ZC0110000 UNII 059QF0KO0R 
Properties
H
2O Molar mass 18.01528(33) g/mol Appearance white solid or almost colorless, transparent, with a slight hint of blue, crystalline solid or liquid[2] Odor odorless Density 999.9720 kg/m3 ≈ 1 t/m3 = 1 kg/l = 1 g/cm3 ≈ 62.4 lb/ft3(liquid, maximum, at ~4 °C)
917 kg/m3 = 0.917 t/m3 = 0.917 kg/l = 0.917 g/cm3 ≈ 57.2 lb/ft3 (solid form) Melting point 0.00 °C (32.00 °F; 273.15 K)[a] Boiling point 100 °C (212 °F; 373 K) [a] Solubility Poorly soluble inhaloalkanes, aliphatic andaromatic hydrocarbons,ethers.[3] Improved solubility in carboxylates, alcohols,ketones, amines. Miscible with methanol, ethanol,isopropanol, acetone,glycerol. Vapor pressure At 25 °C, 0.03127 atm or 3,168 pascals. Acidity (pKa) 13.997 ( at 25 °C)[4][b] Basicity (pKb) 13.997 ( at 25°C)
−1.298·10−5 cm3/mol (20 °C, 1 atm) Thermal conductivity 0.58 W/m·K[6]
Refractive index(nD)
1.3325 Viscosity 1 cP (20 °C) Structure
Hexagonal
C2v
Bent
1.85 D Thermochemistry
75.375 ±0.05 J/mol·K[7][8]
Std molar
entropy (So298)
69.95 J/mol·K[7]
Std enthalpy of
formation (ΔfHo298)
-285.83 kJ/mol[3][7]
Gibbs free energy(ΔfG˚)
-237.24 kJ/mol[3] Hazards Main hazards Drowning
Water intoxication
Avalanche (as snow)
(see also Dihydrogen monoxide hoax) NFPA 704
Flash point Non-flammable Related compounds
Other cations
Hydrogen sulfide
Hydrogen selenide
Hydrogen telluride
Hydrogen polonide
Hydrogen peroxide
Related solvents
Acetone
Methanol
Related compounds
Water vapor
Ice
Heavy water
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
 verify (what is
verify (what is 
 ?)
?)Infobox references
Water (H
2O) is a polar compound that is an important solvent for polar molecules in chemistry, and known as the "universal solvent" for its ability to dissolve many substances.[9] At room temperature, it is a tasteless and odorless liquid, nearly colorless with a hint of blue. It is commonly found in its solid, liquid and gas forms in nature.
Water has hydrogen bonding and is strongly polar. This polarity allows it to separate ions in salts and strongly bond to other polar substances such as alcohols and acids, thus dissolving them. Its hydrogen bonding causes its many unique properties, such as having a solid form less dense than its liquid form, a relatively high boiling point of 100 ºC for its molar mass, and a high heat capacity.
Water is amphoteric, meaning it is both an acid and a base—it produces H+ and OH− ions by self ionization. This regulates the concentrations of H+ and OH− ions in water.
Because water is a very good solvent, water is rarely pure and some properties may vary from those of the pure substance. However, there are also many compounds that are essentially, if not completely, insoluble in water, including fats, oils and other nonpolar substances.
From Wikipedia, the free encyclopedia
"H2O" and "HOH" redirect here. For other uses, see H2O (disambiguation) and HOH (disambiguation).
"Hydrogen monoxide" redirects here. For the prank involving the chemical name of water, see Dihydrogen monoxide hoax.
This article is about the physical and chemical properties of pure water. For general discussion and its distribution and importance in life, see Water. For other uses, seeWater (disambiguation).
 | |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name
water, oxidane
| |||
| Other names
Hydrogen oxide, Dihydrogen monoxide(DHMO), Hydrogen monoxide, Dihydrogen oxide, Hydrogen hydroxide (HH or HOH), Hydric acid, Hydrohydroxic acid, Hydroxic acid, Hydrol,[1] μ-Oxido dihydrogen
| |||
| Identifiers | |||
| 7732-18-5 | |||
| ChEBI | CHEBI:15377 | ||
| ChEMBL | ChEMBL1098659 | ||
| ChemSpider | 937 | ||
| Jmol 3D image | Interactive graph | ||
| PubChem | 962 | ||
| RTECS number | ZC0110000 | ||
| UNII | 059QF0KO0R | ||
| Properties | |||
| H 2O | |||
| Molar mass | 18.01528(33) g/mol | ||
| Appearance | white solid or almost colorless, transparent, with a slight hint of blue, crystalline solid or liquid[2] | ||
| Odor | odorless | ||
| Density | 999.9720 kg/m3 ≈ 1 t/m3 = 1 kg/l = 1 g/cm3 ≈ 62.4 lb/ft3(liquid, maximum, at ~4 °C) 917 kg/m3 = 0.917 t/m3 = 0.917 kg/l = 0.917 g/cm3 ≈ 57.2 lb/ft3 (solid form) | ||
| Melting point | 0.00 °C (32.00 °F; 273.15 K)[a] | ||
| Boiling point | 100 °C (212 °F; 373 K) [a] | ||
| Solubility | Poorly soluble inhaloalkanes, aliphatic andaromatic hydrocarbons,ethers.[3] Improved solubility in carboxylates, alcohols,ketones, amines. Miscible with methanol, ethanol,isopropanol, acetone,glycerol. | ||
| Vapor pressure | At 25 °C, 0.03127 atm or 3,168 pascals. | ||
| Acidity (pKa) | 13.997 ( at 25 °C)[4][b] | ||
| Basicity (pKb) | 13.997 ( at 25°C) | ||
| −1.298·10−5 cm3/mol (20 °C, 1 atm) | |||
| Thermal conductivity | 0.58 W/m·K[6] | ||
Refractive index(nD)
| 1.3325 | ||
| Viscosity | 1 cP (20 °C) | ||
| Structure | |||
| Hexagonal | |||
| C2v | |||
| Bent | |||
| 1.85 D | |||
| Thermochemistry | |||
| 75.375 ±0.05 J/mol·K[7][8] | |||
Std molar
entropy (S | 69.95 J/mol·K[7] | ||
Std enthalpy of
formation (ΔfH | -285.83 kJ/mol[3][7] | ||
Gibbs free energy(ΔfG˚)
| -237.24 kJ/mol[3] | ||
| Hazards | |||
| Main hazards | Drowning Water intoxication Avalanche (as snow) (see also Dihydrogen monoxide hoax) | ||
| NFPA 704 | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
Other cations
| Hydrogen sulfide Hydrogen selenide Hydrogen telluride Hydrogen polonide Hydrogen peroxide | ||
Related solvents
| Acetone Methanol | ||
Related compounds
| Water vapor Ice Heavy water | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
| Infobox references | |||
Water (H
2O) is a polar compound that is an important solvent for polar molecules in chemistry, and known as the "universal solvent" for its ability to dissolve many substances.[9] At room temperature, it is a tasteless and odorless liquid, nearly colorless with a hint of blue. It is commonly found in its solid, liquid and gas forms in nature.
2O) is a polar compound that is an important solvent for polar molecules in chemistry, and known as the "universal solvent" for its ability to dissolve many substances.[9] At room temperature, it is a tasteless and odorless liquid, nearly colorless with a hint of blue. It is commonly found in its solid, liquid and gas forms in nature.
Water has hydrogen bonding and is strongly polar. This polarity allows it to separate ions in salts and strongly bond to other polar substances such as alcohols and acids, thus dissolving them. Its hydrogen bonding causes its many unique properties, such as having a solid form less dense than its liquid form, a relatively high boiling point of 100 ºC for its molar mass, and a high heat capacity.
Water is amphoteric, meaning it is both an acid and a base—it produces H+ and OH− ions by self ionization. This regulates the concentrations of H+ and OH− ions in water.
Because water is a very good solvent, water is rarely pure and some properties may vary from those of the pure substance. However, there are also many compounds that are essentially, if not completely, insoluble in water, including fats, oils and other nonpolar substances.
Contents
[hide]
- 1Forms of water
- 2Physics and chemistry
- 3History
- 4Systematic naming
- 5See also
- 6Notes
- 7References
- 8External links
[hide]
- 1Forms of water
- 2Physics and chemistry
- 3History
- 4Systematic naming
- 5See also
- 6Notes
- 7References
- 8External links
Forms of water
Like many substances, water can take numerous forms that are broadly categorized by phase of matter. The liquid phase is the most common among water's phases (within the Earth's atmosphere and surface) and is the form that is generally denoted by the word "water." The solid phase of water is known as ice and commonly takes the structure of hard, amalgamated crystals, such as ice cubes, or loosely accumulated granular crystals, like snow. For a list of the many different crystalline and amorphous forms of solid ḥ, see the article ice. The gaseous phase of water is known as water vapor (or steam), and is characterized by water assuming the configuration of a transparent cloud. (Note that visible steam and clouds are, in fact, water in the liquid form as minute droplets suspended in the air.) The fourth state of water, that of a supercritical fluid, is much less common than the other three and only rarely occurs in nature, in extremely uninhabitable conditions. When water achieves a specific critical temperature and a specific critical pressure (647 K and 22.064 MPa), liquid and gas phase merge to one homogeneous fluid phase, with properties of both gas and liquid. One example of naturally occurring supercritical water is found in the hottest parts of deep water hydrothermal vents, in which water is heated to the critical temperature by scalding volcanic plumes and achieves the critical pressure because of the crushing weight of the ocean at the extreme depths at which the vents are located. Additionally, anywhere there is volcanic activity below a depth of 2.25 km (1.40 mi) can be expected to have water in the supercritical phase.[c]
Vienna Standard Mean Ocean Water is the current international standard for water isotopes. Naturally occurring water is almost completely composed of the neutron-less hydrogen isotope protium. Only 155 ppm include deuterium (2H or D), a hydrogen isotope with one neutron, and fewer than 20 parts per quintillion include tritium (3H or T), which has two.
Heavy water is water with a higher-than-average deuterium content, up to 100%. Chemically, it is similar but not identical to normal water. This is because the nucleus of deuterium is twice as heavy as protium, and this causes noticeable differences in bonding energies. Because water molecules exchange hydrogen atoms with one another, hydrogen deuterium oxide (DOH) is much more common in low-purity heavy water than pure dideuterium monoxide D
2O. Humans are generally unaware of taste differences,[10] but sometimes report a burning sensation[11] or sweet flavor.[12] Rats, however, are able to avoid heavy water by smell.[13] Toxic to many animals,[13] heavy water is used in the nuclear reactor industry to moderate (slow down) neutrons. Light water reactors are also common, where "light" simply designates normal water.
Light water more specifically refers to deuterium-depleted water (DDW), water in which the deuterium content has been reduced below the standard 155 ppm level.
Like many substances, water can take numerous forms that are broadly categorized by phase of matter. The liquid phase is the most common among water's phases (within the Earth's atmosphere and surface) and is the form that is generally denoted by the word "water." The solid phase of water is known as ice and commonly takes the structure of hard, amalgamated crystals, such as ice cubes, or loosely accumulated granular crystals, like snow. For a list of the many different crystalline and amorphous forms of solid ḥ, see the article ice. The gaseous phase of water is known as water vapor (or steam), and is characterized by water assuming the configuration of a transparent cloud. (Note that visible steam and clouds are, in fact, water in the liquid form as minute droplets suspended in the air.) The fourth state of water, that of a supercritical fluid, is much less common than the other three and only rarely occurs in nature, in extremely uninhabitable conditions. When water achieves a specific critical temperature and a specific critical pressure (647 K and 22.064 MPa), liquid and gas phase merge to one homogeneous fluid phase, with properties of both gas and liquid. One example of naturally occurring supercritical water is found in the hottest parts of deep water hydrothermal vents, in which water is heated to the critical temperature by scalding volcanic plumes and achieves the critical pressure because of the crushing weight of the ocean at the extreme depths at which the vents are located. Additionally, anywhere there is volcanic activity below a depth of 2.25 km (1.40 mi) can be expected to have water in the supercritical phase.[c]
Vienna Standard Mean Ocean Water is the current international standard for water isotopes. Naturally occurring water is almost completely composed of the neutron-less hydrogen isotope protium. Only 155 ppm include deuterium (2H or D), a hydrogen isotope with one neutron, and fewer than 20 parts per quintillion include tritium (3H or T), which has two.
Heavy water is water with a higher-than-average deuterium content, up to 100%. Chemically, it is similar but not identical to normal water. This is because the nucleus of deuterium is twice as heavy as protium, and this causes noticeable differences in bonding energies. Because water molecules exchange hydrogen atoms with one another, hydrogen deuterium oxide (DOH) is much more common in low-purity heavy water than pure dideuterium monoxide D
2O. Humans are generally unaware of taste differences,[10] but sometimes report a burning sensation[11] or sweet flavor.[12] Rats, however, are able to avoid heavy water by smell.[13] Toxic to many animals,[13] heavy water is used in the nuclear reactor industry to moderate (slow down) neutrons. Light water reactors are also common, where "light" simply designates normal water.
2O. Humans are generally unaware of taste differences,[10] but sometimes report a burning sensation[11] or sweet flavor.[12] Rats, however, are able to avoid heavy water by smell.[13] Toxic to many animals,[13] heavy water is used in the nuclear reactor industry to moderate (slow down) neutrons. Light water reactors are also common, where "light" simply designates normal water.
Light water more specifically refers to deuterium-depleted water (DDW), water in which the deuterium content has been reduced below the standard 155 ppm level.
Physics and chemistry
See also: Water chemistry analysis
Water is the chemical substance with chemical formula H
2O; one molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom.[14] Water is a tasteless, odorless liquid at ambient temperature and pressure, and appears colorless in small quantities, although it has its own intrinsic very light blue hue. Ice also appears colorless, and water vapor is essentially invisible as a gas.[2]
Water is primarily a liquid under standard conditions, which is not predicted from its relationship to other analogous hydrides of theoxygen family in the periodic table, which are gases such as hydrogen sulfide. The elements surrounding oxygen in the periodic table, nitrogen, fluorine, phosphorus, sulfur and chlorine, all combine with hydrogen to produce gases under standard conditions. The reason that water forms a liquid is that oxygen is more electronegative than all of these elements with the exception of fluorine. Oxygen attracts electrons much more strongly than hydrogen, resulting in a net positive charge on the hydrogen atoms, and a net negative charge on the oxygen atom. The presence of a charge on each of these atoms gives each water molecule a net dipole moment. Electrical attraction between water molecules due to this dipole pulls individual molecules closer together, making it more difficult to separate the molecules and therefore raising the boiling point. This attraction is known as hydrogen bonding. The molecules of water are constantly moving in relation to each other, and the hydrogen bonds are continually breaking and reforming at timescales faster than 200 femtoseconds.[15] However, this bond is sufficiently strong to create many of the peculiar properties of water, such as those that make it integral to life. Water can be described as a polar liquid that slightly dissociates disproportionately into the hydronium ion (H
3O+(aq)) and an associated hydroxide ion (OH−(aq)).
- 2 H
2O(l) ⇌ H
3O+(aq) + OH−(aq)
The dissociation constant for this dissociation is commonly symbolized as Kw and has a value of about 10−14 at 25 °C; see "Water (data page)" and "Self-ionization of water" for more information.
Percentage of elements in water by mass: 11.1% hydrogen, 88.9% oxygen.
The self-diffusion coefficient of water is 2.299·10−9 m2·s−1.[16]
See also: Water chemistry analysis
Water is the chemical substance with chemical formula H
2O; one molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom.[14] Water is a tasteless, odorless liquid at ambient temperature and pressure, and appears colorless in small quantities, although it has its own intrinsic very light blue hue. Ice also appears colorless, and water vapor is essentially invisible as a gas.[2]
2O; one molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom.[14] Water is a tasteless, odorless liquid at ambient temperature and pressure, and appears colorless in small quantities, although it has its own intrinsic very light blue hue. Ice also appears colorless, and water vapor is essentially invisible as a gas.[2]
Water is primarily a liquid under standard conditions, which is not predicted from its relationship to other analogous hydrides of theoxygen family in the periodic table, which are gases such as hydrogen sulfide. The elements surrounding oxygen in the periodic table, nitrogen, fluorine, phosphorus, sulfur and chlorine, all combine with hydrogen to produce gases under standard conditions. The reason that water forms a liquid is that oxygen is more electronegative than all of these elements with the exception of fluorine. Oxygen attracts electrons much more strongly than hydrogen, resulting in a net positive charge on the hydrogen atoms, and a net negative charge on the oxygen atom. The presence of a charge on each of these atoms gives each water molecule a net dipole moment. Electrical attraction between water molecules due to this dipole pulls individual molecules closer together, making it more difficult to separate the molecules and therefore raising the boiling point. This attraction is known as hydrogen bonding. The molecules of water are constantly moving in relation to each other, and the hydrogen bonds are continually breaking and reforming at timescales faster than 200 femtoseconds.[15] However, this bond is sufficiently strong to create many of the peculiar properties of water, such as those that make it integral to life. Water can be described as a polar liquid that slightly dissociates disproportionately into the hydronium ion (H
3O+(aq)) and an associated hydroxide ion (OH−(aq)).
3O+(aq)) and an associated hydroxide ion (OH−(aq)).
- 2 H
2O(l) ⇌ H
3O+(aq) + OH−(aq)
The dissociation constant for this dissociation is commonly symbolized as Kw and has a value of about 10−14 at 25 °C; see "Water (data page)" and "Self-ionization of water" for more information.
Percentage of elements in water by mass: 11.1% hydrogen, 88.9% oxygen.
The self-diffusion coefficient of water is 2.299·10−9 m2·s−1.[16]
Water, ice, and vapor
Heat capacity and heats of vaporization and fusion
Heat of vaporization
Temperature (°C)  Hvap (kJ/mol)[17]
Hvap (kJ/mol)[17]0 45.054 25 43.99 40 43.35 60 42.482 80 41.585 100 40.657 120 39.684 140 38.643 160 37.518 180 36.304 200 34.962 220 33.468 240 31.809 260 29.93 280 27.795 300 25.3 320 22.297 340 18.502 360 12.966 374 2.066
Main articles: Enthalpy of vaporization and Enthalpy of fusion
Water has a very high specific heat capacity – the second highest among all the heteroatomic species (after ammonia), as well as a high heat of vaporization (40.65 kJ/mol or 2257 kJ/kg at the normal boiling point), both of which are a result of the extensive hydrogen bonding between its molecules. These two unusual properties allow water to moderate Earth's climate by buffering large fluctuations in temperature. According to Josh Willis, of NASA's Jet Propulsion Laboratory, the oceans absorb one thousand times more heat than the atmosphere (air) and are holding 80 to 90% of the heat of global warming.[18]
The specific enthalpy of fusion of water is 333.55 kJ/kg at 0 °C, i.e. melting ice absorbs the same energy as ice warming from -160 degrees Celsius up to its melting point. Similarly the heat needed to melt ice at 0 °C, would heat the same amount of water by about 80 °C. Of common substances, only that of ammonia is higher. This property confers resistance to melting on the ice of glaciers and drift ice. Before and since the advent of mechanicalrefrigeration, ice was and still is in common use for retarding food spoilage.
Constant-pressure heat capacity
Temperature (°C) Cp (J/(g·K) at 100 kPa)[8] 0 4.2176 10 4.1921 20 4.1818 25 4.1814 30 4.1784 40 4.1785 50 4.1806 60 4.1843 70 4.1895 80 4.1963 90 4.205 100 4.2159
Note that the specific heat capacity of ice at −10 °C is about 2.05 J/(g·K) and that the heat capacity of steam at 100 °C is about 2.080 J/(g·K).
| Temperature (°C) |  Hvap (kJ/mol)[17] Hvap (kJ/mol)[17] |
|---|---|
| 0 | 45.054 |
| 25 | 43.99 |
| 40 | 43.35 |
| 60 | 42.482 |
| 80 | 41.585 |
| 100 | 40.657 |
| 120 | 39.684 |
| 140 | 38.643 |
| 160 | 37.518 |
| 180 | 36.304 |
| 200 | 34.962 |
| 220 | 33.468 |
| 240 | 31.809 |
| 260 | 29.93 |
| 280 | 27.795 |
| 300 | 25.3 |
| 320 | 22.297 |
| 340 | 18.502 |
| 360 | 12.966 |
| 374 | 2.066 |
Main articles: Enthalpy of vaporization and Enthalpy of fusion
Water has a very high specific heat capacity – the second highest among all the heteroatomic species (after ammonia), as well as a high heat of vaporization (40.65 kJ/mol or 2257 kJ/kg at the normal boiling point), both of which are a result of the extensive hydrogen bonding between its molecules. These two unusual properties allow water to moderate Earth's climate by buffering large fluctuations in temperature. According to Josh Willis, of NASA's Jet Propulsion Laboratory, the oceans absorb one thousand times more heat than the atmosphere (air) and are holding 80 to 90% of the heat of global warming.[18]
The specific enthalpy of fusion of water is 333.55 kJ/kg at 0 °C, i.e. melting ice absorbs the same energy as ice warming from -160 degrees Celsius up to its melting point. Similarly the heat needed to melt ice at 0 °C, would heat the same amount of water by about 80 °C. Of common substances, only that of ammonia is higher. This property confers resistance to melting on the ice of glaciers and drift ice. Before and since the advent of mechanicalrefrigeration, ice was and still is in common use for retarding food spoilage.
| Temperature (°C) | Cp (J/(g·K) at 100 kPa)[8] |
|---|---|
| 0 | 4.2176 |
| 10 | 4.1921 |
| 20 | 4.1818 |
| 25 | 4.1814 |
| 30 | 4.1784 |
| 40 | 4.1785 |
| 50 | 4.1806 |
| 60 | 4.1843 |
| 70 | 4.1895 |
| 80 | 4.1963 |
| 90 | 4.205 |
| 100 | 4.2159 |
Note that the specific heat capacity of ice at −10 °C is about 2.05 J/(g·K) and that the heat capacity of steam at 100 °C is about 2.080 J/(g·K).
Density of water and ice
Density of liquid water
Temp (°C) Density (kg/m3)[19][20] +100 958.4 +80 971.8 +60 983.2 +40 992.2 +30 995.6502 +25 997.0479 +22 997.7735 +20 998.2071 +15 999.1026 +10 999.7026 +4 999.9720 0 999.8395 −10 998.117 −20 993.547 −30 983.854 The values below 0 °C refer to supercooled water.
The density of water is approximately one gram per cubic centimeter (62.43 lb./ft.³). It is dependent on its temperature, but the relation is not linear and is unimodal rather thanmonotonic (see table at left). When cooled from room temperature liquid water becomes increasingly dense, as with other substances, but at approximately 4 °C (39 °F), pure water reaches its maximum density. As it is cooled further, it expands to become less dense. This unusual negative thermal expansion is attributed to strong, orientation-dependent, intermolecular interactions and is also observed in molten silica.[21]
The solid form of most substances is denser than the liquid phase; thus, a block of most solids will sink in the liquid. However, a block of ice floats in liquid water because ice is less dense. Upon freezing, the density of water decreases by about 9%.[22] This is due to the 'cooling' of intermolecular vibrations allowing the molecules to form steady hydrogen bonds with their neighbors and thereby gradually locking into positions reminiscent of the hexagonal packing achieved upon freezing to ice Ih. Whereas the hydrogen bonds are shorter in the crystal than in the liquid, this locking effect reduces the average coordination number of molecules as the liquid approaches nucleation. Other substances that expand on freezing are acetic acid, silicon, gallium, germanium, antimony, bismuth, plutonium and also chemical compounds that form spacious crystal lattices with tetrahedral coordination.
Only ordinary hexagonal ice is less dense than the liquid. Under increasing pressure, ice undergoes a number of transitions to otherallotropic forms with higher density than liquid water, such as ice II, ice III, high-density amorphous ice (HDA), and very-high-density amorphous ice (VHDA).
Water also expands significantly as the temperature increases. Water near the boiling point is about 96% as dense as water at 4 °C (39.2°F).
The melting point of ice is 0 °C (32 °F, 273.15 K) at standard pressure, however, pure liquid water can be supercooled well below that temperature without freezing if the liquid is not mechanically disturbed. It can remain in a fluid state down to its homogeneousnucleation point of approximately 231 K (−42 °C, -43.87°F).[23] The melting point of ordinary hexagonal ice falls slightly under moderately high pressures, by 0.0073 °C (0.0131 °F)/atm[d] or about 0.5 °C (0.90 °F)/70 atm[e][24] as the stabilization energy of hydrogen bonding is exceeded by intermolecular repulsion, but as ice transforms into itsallotropes (see crystalline states of ice) above 209.9 MPa (2,072 atm), the melting point increases markedly with pressure, i.e., reaching 355 K (82 °C) at 2.216 GPa (21,870 atm) (triple point of Ice VII[25]).
A significant increase of pressure is required to lower the melting point of ordinary ice—the pressure exerted by an ice skater on the ice only reduces the melting point by approximately 0.09 °C (0.16 °F).[citation needed]
These properties of water have important consequences in its role in Earth's ecosystem. Water at a temperature of 4 °C (39.2 °F) will always accumulate at the bottom of freshwater lakes, irrespective of the temperature in the atmosphere.
In cold countries, when the temperature of fresh water reaches 4 °C, the layers of water near the top in contact with cold air continue to lose heat energy and their temperature falls below 4 °C. On cooling below 4 °C, these layers do not sink as fresh water has a maximum density at 4 °C. (Refer: Polarity and hydrogen bonding) Due to this, the layer of water at 4 °C remains at the bottom and above this layers of water 3 °C, 2 °C, 1 °C and 0 °C are formed. As water at 0 °C is the least dense it floats on the top and turns into ice as the water continues to cool. Ice growth continues on the bottom of the ice as heat is drawn away through the ice (the heat conductivity of ice is similar to glass). All the while the water further down below the ice is still 4 °C. As the ice layer shields the lake from the effect of the wind, water in the lake will no longer turn over. Although both water and ice are relatively good conductors of heat, a thick layer of ice and a thick layer of stratified water under the ice slow down further heat loss from the lake relative to when the lake was exposed. It is, therefore, unlikely that sufficiently deep lakes will freeze completely, unless stirred by strong currents that mix cooler and warmer water and accelerate the cooling. Thus, as long as the pond or lake does not freeze up completely, aquatic creatures are not exposed to freezing temperatures. In warming weather, chunks of ice float, rather than sink to the bottom where they might melt extremely slowly. These properties therefore allow aquatic life in the lake to survive during the winter.
| Temp (°C) | Density (kg/m3)[19][20] |
|---|---|
| +100 | 958.4 |
| +80 | 971.8 |
| +60 | 983.2 |
| +40 | 992.2 |
| +30 | 995.6502 |
| +25 | 997.0479 |
| +22 | 997.7735 |
| +20 | 998.2071 |
| +15 | 999.1026 |
| +10 | 999.7026 |
| +4 | 999.9720 |
| 0 | 999.8395 |
| −10 | 998.117 |
| −20 | 993.547 |
| −30 | 983.854 |
| The values below 0 °C refer to supercooled water. | |
The density of water is approximately one gram per cubic centimeter (62.43 lb./ft.³). It is dependent on its temperature, but the relation is not linear and is unimodal rather thanmonotonic (see table at left). When cooled from room temperature liquid water becomes increasingly dense, as with other substances, but at approximately 4 °C (39 °F), pure water reaches its maximum density. As it is cooled further, it expands to become less dense. This unusual negative thermal expansion is attributed to strong, orientation-dependent, intermolecular interactions and is also observed in molten silica.[21]
The solid form of most substances is denser than the liquid phase; thus, a block of most solids will sink in the liquid. However, a block of ice floats in liquid water because ice is less dense. Upon freezing, the density of water decreases by about 9%.[22] This is due to the 'cooling' of intermolecular vibrations allowing the molecules to form steady hydrogen bonds with their neighbors and thereby gradually locking into positions reminiscent of the hexagonal packing achieved upon freezing to ice Ih. Whereas the hydrogen bonds are shorter in the crystal than in the liquid, this locking effect reduces the average coordination number of molecules as the liquid approaches nucleation. Other substances that expand on freezing are acetic acid, silicon, gallium, germanium, antimony, bismuth, plutonium and also chemical compounds that form spacious crystal lattices with tetrahedral coordination.
Only ordinary hexagonal ice is less dense than the liquid. Under increasing pressure, ice undergoes a number of transitions to otherallotropic forms with higher density than liquid water, such as ice II, ice III, high-density amorphous ice (HDA), and very-high-density amorphous ice (VHDA).
Water also expands significantly as the temperature increases. Water near the boiling point is about 96% as dense as water at 4 °C (39.2°F).
The melting point of ice is 0 °C (32 °F, 273.15 K) at standard pressure, however, pure liquid water can be supercooled well below that temperature without freezing if the liquid is not mechanically disturbed. It can remain in a fluid state down to its homogeneousnucleation point of approximately 231 K (−42 °C, -43.87°F).[23] The melting point of ordinary hexagonal ice falls slightly under moderately high pressures, by 0.0073 °C (0.0131 °F)/atm[d] or about 0.5 °C (0.90 °F)/70 atm[e][24] as the stabilization energy of hydrogen bonding is exceeded by intermolecular repulsion, but as ice transforms into itsallotropes (see crystalline states of ice) above 209.9 MPa (2,072 atm), the melting point increases markedly with pressure, i.e., reaching 355 K (82 °C) at 2.216 GPa (21,870 atm) (triple point of Ice VII[25]).
A significant increase of pressure is required to lower the melting point of ordinary ice—the pressure exerted by an ice skater on the ice only reduces the melting point by approximately 0.09 °C (0.16 °F).[citation needed]
These properties of water have important consequences in its role in Earth's ecosystem. Water at a temperature of 4 °C (39.2 °F) will always accumulate at the bottom of freshwater lakes, irrespective of the temperature in the atmosphere.
In cold countries, when the temperature of fresh water reaches 4 °C, the layers of water near the top in contact with cold air continue to lose heat energy and their temperature falls below 4 °C. On cooling below 4 °C, these layers do not sink as fresh water has a maximum density at 4 °C. (Refer: Polarity and hydrogen bonding) Due to this, the layer of water at 4 °C remains at the bottom and above this layers of water 3 °C, 2 °C, 1 °C and 0 °C are formed. As water at 0 °C is the least dense it floats on the top and turns into ice as the water continues to cool. Ice growth continues on the bottom of the ice as heat is drawn away through the ice (the heat conductivity of ice is similar to glass). All the while the water further down below the ice is still 4 °C. As the ice layer shields the lake from the effect of the wind, water in the lake will no longer turn over. Although both water and ice are relatively good conductors of heat, a thick layer of ice and a thick layer of stratified water under the ice slow down further heat loss from the lake relative to when the lake was exposed. It is, therefore, unlikely that sufficiently deep lakes will freeze completely, unless stirred by strong currents that mix cooler and warmer water and accelerate the cooling. Thus, as long as the pond or lake does not freeze up completely, aquatic creatures are not exposed to freezing temperatures. In warming weather, chunks of ice float, rather than sink to the bottom where they might melt extremely slowly. These properties therefore allow aquatic life in the lake to survive during the winter.
Density of saltwater and ice
The density of water is dependent on the dissolved salt content as well as the temperature of the water. Ice still floats in the oceans, otherwise they would freeze from the bottom up. However, the salt content of oceans lowers the freezing point by about 2 °C (see here for explanation) and lowers the temperature of the density maximum of water to the freezing point. This is why, in ocean water, the downward convection of colder water is not blocked by an expansion of water as it becomes colder near the freezing point. The oceans' cold water near the freezing point continues to sink. For this reason, any creature attempting to survive at the bottom of such cold water as the Arctic Ocean generally lives in water that is 4 °C colder than the temperature at the bottom of frozen-over fresh water lakes and rivers in the winter.
As the surface of salt water begins to freeze (at −1.9 °C for normal salinity seawater, 3.5%) the ice that forms is essentially salt free with a density approximately equal to that of freshwater ice. This ice floats on the surface and the salt that is "frozen out" adds to the salinity and density of the seawater just below it, in a process known as brine rejection. This denser saltwater sinks by convection and the replacing seawater is subject to the same process. This provides essentially freshwater ice at −1.9 °C on the surface. The increased density of the seawater beneath the forming ice causes it to sink towards the bottom. On a large scale, the process of brine rejection and sinking cold salty water results in ocean currents forming to transport such water away from the Poles, leading to a global system of currents called the thermohaline circulation.
The density of water is dependent on the dissolved salt content as well as the temperature of the water. Ice still floats in the oceans, otherwise they would freeze from the bottom up. However, the salt content of oceans lowers the freezing point by about 2 °C (see here for explanation) and lowers the temperature of the density maximum of water to the freezing point. This is why, in ocean water, the downward convection of colder water is not blocked by an expansion of water as it becomes colder near the freezing point. The oceans' cold water near the freezing point continues to sink. For this reason, any creature attempting to survive at the bottom of such cold water as the Arctic Ocean generally lives in water that is 4 °C colder than the temperature at the bottom of frozen-over fresh water lakes and rivers in the winter.
As the surface of salt water begins to freeze (at −1.9 °C for normal salinity seawater, 3.5%) the ice that forms is essentially salt free with a density approximately equal to that of freshwater ice. This ice floats on the surface and the salt that is "frozen out" adds to the salinity and density of the seawater just below it, in a process known as brine rejection. This denser saltwater sinks by convection and the replacing seawater is subject to the same process. This provides essentially freshwater ice at −1.9 °C on the surface. The increased density of the seawater beneath the forming ice causes it to sink towards the bottom. On a large scale, the process of brine rejection and sinking cold salty water results in ocean currents forming to transport such water away from the Poles, leading to a global system of currents called the thermohaline circulation.
Miscibility and condensation
Main article: Humidity
See also: List of water-miscible solvents
Water is miscible with many liquids, for example ethanol in all proportions, forming a single homogeneous liquid. On the other hand, water and most oils are immiscible usually forming layers according to increasing density from the top. This can be predicted by comparing the polarity. Water being a relatively polar compound will tend to be miscible with liquids of high polarity such as ethanol and acetone whereas compounds with low polarity will tend to be immiscible and poorly soluble such as with hydrocarbons.
As a gas, water vapor is completely miscible with air. On the other hand, the maximum water vapor pressure that is thermodynamically stable with the liquid (or solid) at a given temperature is relatively low compared with total atmospheric pressure. For example, if the vapor partial pressure[f] is 2% of atmospheric pressure and the air is cooled from 25 °C, starting at about 22 °C water will start to condense, defining the dew point, and creating fog or dew. The reverse process accounts for the fog burning off in the morning. If the humidity is increased at room temperature, for example, by running a hot shower or a bath, and the temperature stays about the same, the vapor soon reaches the pressure for phase change, and then condenses out as minute water droplets, commonly referred to as steam.
A gas in this context is referred to as saturated or 100% relative humidity, when the vapor pressure of water in the air is at the equilibrium with vapor pressure due to (liquid) water; water (or ice, if cool enough) will fail to lose mass through evaporation when exposed to saturated air. Because the amount of water vapor in air is small, relative humidity, the ratio of the partial pressure due to the water vapor to the saturated partial vapor pressure, is much more useful. Water vapor pressure above 100% relative humidity is called super-saturatedand can occur if air is rapidly cooled, for example, by rising suddenly in an updraft.[g]
Main article: Humidity
See also: List of water-miscible solvents
Water is miscible with many liquids, for example ethanol in all proportions, forming a single homogeneous liquid. On the other hand, water and most oils are immiscible usually forming layers according to increasing density from the top. This can be predicted by comparing the polarity. Water being a relatively polar compound will tend to be miscible with liquids of high polarity such as ethanol and acetone whereas compounds with low polarity will tend to be immiscible and poorly soluble such as with hydrocarbons.
As a gas, water vapor is completely miscible with air. On the other hand, the maximum water vapor pressure that is thermodynamically stable with the liquid (or solid) at a given temperature is relatively low compared with total atmospheric pressure. For example, if the vapor partial pressure[f] is 2% of atmospheric pressure and the air is cooled from 25 °C, starting at about 22 °C water will start to condense, defining the dew point, and creating fog or dew. The reverse process accounts for the fog burning off in the morning. If the humidity is increased at room temperature, for example, by running a hot shower or a bath, and the temperature stays about the same, the vapor soon reaches the pressure for phase change, and then condenses out as minute water droplets, commonly referred to as steam.
A gas in this context is referred to as saturated or 100% relative humidity, when the vapor pressure of water in the air is at the equilibrium with vapor pressure due to (liquid) water; water (or ice, if cool enough) will fail to lose mass through evaporation when exposed to saturated air. Because the amount of water vapor in air is small, relative humidity, the ratio of the partial pressure due to the water vapor to the saturated partial vapor pressure, is much more useful. Water vapor pressure above 100% relative humidity is called super-saturatedand can occur if air is rapidly cooled, for example, by rising suddenly in an updraft.[g]
Vapor pressure
Main article: Vapor pressure of water
Temperature Pressure[26] °C K °F Pa atm torr(mmHg) in Hg psi 0 273 32 611 0.00603 4.58 0.180 0.0886 5 278 41 872 0.00861 6.54 0.257 0.1265 10 283 50 1,228 0.01212 9.21 0.363 0.1781 12 285 54 1,403 0.01385 10.52 0.414 0.2034 14 287 57 1,599 0.01578 11.99 0.472 0.2318 16 289 61 1,817 0.01793 13.63 0.537 0.2636 17 290 63 1,937 0.01912 14.53 0.572 0.2810 18 291 64 2,064 0.02037 15.48 0.609 0.2993 19 292 66 2,197 0.02168 16.48 0.649 0.3187 20 293 68 2,338 0.02307 17.54 0.691 0.3392 21 294 70 2,486 0.02453 18.65 0.734 0.3606 22 295 72 2,644 0.02609 19.83 0.781 0.3834 23 296 73 2,809 0.02772 21.07 0.830 0.4074 24 297 75 2,984 0.02945 22.38 0.881 0.4328 25 298 77 3,168 0.03127 23.76 0.935 0.4594
Main article: Vapor pressure of water
| Temperature | Pressure[26] | ||||||
|---|---|---|---|---|---|---|---|
| °C | K | °F | Pa | atm | torr(mmHg) | in Hg | psi |
| 0 | 273 | 32 | 611 | 0.00603 | 4.58 | 0.180 | 0.0886 |
| 5 | 278 | 41 | 872 | 0.00861 | 6.54 | 0.257 | 0.1265 |
| 10 | 283 | 50 | 1,228 | 0.01212 | 9.21 | 0.363 | 0.1781 |
| 12 | 285 | 54 | 1,403 | 0.01385 | 10.52 | 0.414 | 0.2034 |
| 14 | 287 | 57 | 1,599 | 0.01578 | 11.99 | 0.472 | 0.2318 |
| 16 | 289 | 61 | 1,817 | 0.01793 | 13.63 | 0.537 | 0.2636 |
| 17 | 290 | 63 | 1,937 | 0.01912 | 14.53 | 0.572 | 0.2810 |
| 18 | 291 | 64 | 2,064 | 0.02037 | 15.48 | 0.609 | 0.2993 |
| 19 | 292 | 66 | 2,197 | 0.02168 | 16.48 | 0.649 | 0.3187 |
| 20 | 293 | 68 | 2,338 | 0.02307 | 17.54 | 0.691 | 0.3392 |
| 21 | 294 | 70 | 2,486 | 0.02453 | 18.65 | 0.734 | 0.3606 |
| 22 | 295 | 72 | 2,644 | 0.02609 | 19.83 | 0.781 | 0.3834 |
| 23 | 296 | 73 | 2,809 | 0.02772 | 21.07 | 0.830 | 0.4074 |
| 24 | 297 | 75 | 2,984 | 0.02945 | 22.38 | 0.881 | 0.4328 |
| 25 | 298 | 77 | 3,168 | 0.03127 | 23.76 | 0.935 | 0.4594 |
Compressibility
The compressibility of water is a function of pressure and temperature. At 0 °C, at the limit of zero pressure, the compressibility is 5.1×10−10 Pa−1.[27] At the zero-pressure limit, the compressibility reaches a minimum of 4.4×10−10 Pa−1 around 45 °C before increasing again with increasing temperature. As the pressure is increased, the compressibility decreases, being 3.9×10−10 Pa−1 at 0 °C and 100 MPa.
The bulk modulus of water is about 2.2 GPa.[28] The low compressibility of non-gases, and of water in particular, leads to their often being assumed as incompressible. The low compressibility of water means that even in the deep oceans at 4 km depth, where pressures are 40 MPa, there is only a 1.8% decrease in volume.[28]
The compressibility of water is a function of pressure and temperature. At 0 °C, at the limit of zero pressure, the compressibility is 5.1×10−10 Pa−1.[27] At the zero-pressure limit, the compressibility reaches a minimum of 4.4×10−10 Pa−1 around 45 °C before increasing again with increasing temperature. As the pressure is increased, the compressibility decreases, being 3.9×10−10 Pa−1 at 0 °C and 100 MPa.
The bulk modulus of water is about 2.2 GPa.[28] The low compressibility of non-gases, and of water in particular, leads to their often being assumed as incompressible. The low compressibility of water means that even in the deep oceans at 4 km depth, where pressures are 40 MPa, there is only a 1.8% decrease in volume.[28]
Triple point
The various triple points of water
Phases in stable equilibrium Pressure Temperature liquid water, ice Ih, and water vapor 611.657 Pa[29] [30] 273.16 K (0.01 °C) liquid water, ice Ih, and ice III 209.9 MPa 251 K (−22 °C) liquid water, ice III, and ice V 350.1 MPa −17.0 °C liquid water, ice V, and ice VI 632.4 MPa 0.16 °C ice Ih, Ice II, and ice III 213 MPa −35 °C ice II, ice III, and ice V 344 MPa −24 °C ice II, ice V, and ice VI 626 MPa −70 °C
The temperature and pressure at which solid, liquid, and gaseous water coexist in equilibrium is called the triple point of water. This point is used to define the units of temperature (the kelvin, the SI unit of thermodynamic temperature and, indirectly, the degree Celsius and even the degreeFahrenheit).
As a consequence, water's triple point temperature, as measured in these units, is a prescribed value rather than a measured quantity.
This pressure is quite low, about 1⁄166 of the normal sea level barometric pressure of 101,325 Pa. The atmospheric surface pressure on planet Mars is 610.5 Pa, which is remarkably close to the triple point pressure. The altitude of this surface pressure was used to define zero-elevation or "sea level" on that planet.[31]
Although it is commonly named as "the triple point of water", the stable combination of liquid water, ice I, and water vapor is but one of several triple points on the phase diagram of water. Gustav Heinrich Johann Apollon Tammann in Göttingen produced data on several other triple points in the early 20th century. Kamb and others documented further triple points in the 1960s.[32][33][34]
| Phases in stable equilibrium | Pressure | Temperature |
|---|---|---|
| liquid water, ice Ih, and water vapor | 611.657 Pa[29] [30] | 273.16 K (0.01 °C) |
| liquid water, ice Ih, and ice III | 209.9 MPa | 251 K (−22 °C) |
| liquid water, ice III, and ice V | 350.1 MPa | −17.0 °C |
| liquid water, ice V, and ice VI | 632.4 MPa | 0.16 °C |
| ice Ih, Ice II, and ice III | 213 MPa | −35 °C |
| ice II, ice III, and ice V | 344 MPa | −24 °C |
| ice II, ice V, and ice VI | 626 MPa | −70 °C |
The temperature and pressure at which solid, liquid, and gaseous water coexist in equilibrium is called the triple point of water. This point is used to define the units of temperature (the kelvin, the SI unit of thermodynamic temperature and, indirectly, the degree Celsius and even the degreeFahrenheit).
As a consequence, water's triple point temperature, as measured in these units, is a prescribed value rather than a measured quantity.
This pressure is quite low, about 1⁄166 of the normal sea level barometric pressure of 101,325 Pa. The atmospheric surface pressure on planet Mars is 610.5 Pa, which is remarkably close to the triple point pressure. The altitude of this surface pressure was used to define zero-elevation or "sea level" on that planet.[31]
Although it is commonly named as "the triple point of water", the stable combination of liquid water, ice I, and water vapor is but one of several triple points on the phase diagram of water. Gustav Heinrich Johann Apollon Tammann in Göttingen produced data on several other triple points in the early 20th century. Kamb and others documented further triple points in the 1960s.[32][33][34]
Electrical properties
Electrical conductivity
Pure water containing no exogenous ions is an excellent insulator, but not even "deionized" water is completely free of ions. Water undergoes auto-ionization in the liquid state, when two water molecules form one hydroxide anion (OH− ) and one hydronium cation (H
3O+ ).
Because water is such a good solvent, it almost always has some solute dissolved in it, often a salt. If water has even a tiny amount of such an impurity, then it can conduct electricity far more readily.
It is known that the theoretical maximum electrical resistivity for water is approximately 182 kΩ·m at 25 °C. This figure agrees well with what is typically seen on reverse osmosis, ultra-filtered and deionized ultra-pure water systems used, for instance, in semiconductor manufacturing plants. A salt or acid contaminant level exceeding even 100 parts per trillion (ppt) in otherwise ultra-pure water begins to noticeably lower its resistivity by up to several kΩ·m.[citation needed]
In pure water, sensitive equipment can detect a very slight electrical conductivity of 0.055 µS/cm at 25 °C. Water can also be electrolyzed into oxygen and hydrogen gases but in the absence of dissolved ions this is a very slow process, as very little current is conducted. In ice, the primary charge carriers are protons (see proton conductor).[35]
Pure water containing no exogenous ions is an excellent insulator, but not even "deionized" water is completely free of ions. Water undergoes auto-ionization in the liquid state, when two water molecules form one hydroxide anion (OH− ) and one hydronium cation (H
3O+ ).
3O+ ).
Because water is such a good solvent, it almost always has some solute dissolved in it, often a salt. If water has even a tiny amount of such an impurity, then it can conduct electricity far more readily.
It is known that the theoretical maximum electrical resistivity for water is approximately 182 kΩ·m at 25 °C. This figure agrees well with what is typically seen on reverse osmosis, ultra-filtered and deionized ultra-pure water systems used, for instance, in semiconductor manufacturing plants. A salt or acid contaminant level exceeding even 100 parts per trillion (ppt) in otherwise ultra-pure water begins to noticeably lower its resistivity by up to several kΩ·m.[citation needed]
In pure water, sensitive equipment can detect a very slight electrical conductivity of 0.055 µS/cm at 25 °C. Water can also be electrolyzed into oxygen and hydrogen gases but in the absence of dissolved ions this is a very slow process, as very little current is conducted. In ice, the primary charge carriers are protons (see proton conductor).[35]
Electrolysis
Main article: Electrolysis of water
Water can be split into its constituent elements, hydrogen and oxygen, by passing an electric current through it. This process is called electrolysis. Water molecules dissociate intoH+ and OH− ions, which are attracted toward the cathode and anode, respectively. At the cathode, two H+ ions pick up electrons and form H
2 gas. At the anode, four OH− ions combine and release O
2 gas, molecular water, and four electrons. The gases produced bubble to the surface, where they can be collected. The standard potential of the water electrolysis cell (when heat is added to the reaction) is a minimum of 1.23 V at 25 °C. The operating potential is actually 1.48 V (or above) in practical electrolysis when heat input is negligible.
Main article: Electrolysis of water
Water can be split into its constituent elements, hydrogen and oxygen, by passing an electric current through it. This process is called electrolysis. Water molecules dissociate intoH+ and OH− ions, which are attracted toward the cathode and anode, respectively. At the cathode, two H+ ions pick up electrons and form H
2 gas. At the anode, four OH− ions combine and release O
2 gas, molecular water, and four electrons. The gases produced bubble to the surface, where they can be collected. The standard potential of the water electrolysis cell (when heat is added to the reaction) is a minimum of 1.23 V at 25 °C. The operating potential is actually 1.48 V (or above) in practical electrolysis when heat input is negligible.
2 gas. At the anode, four OH− ions combine and release O
2 gas, molecular water, and four electrons. The gases produced bubble to the surface, where they can be collected. The standard potential of the water electrolysis cell (when heat is added to the reaction) is a minimum of 1.23 V at 25 °C. The operating potential is actually 1.48 V (or above) in practical electrolysis when heat input is negligible.
Static dielectric constant
dielectric constant of water [36]
temperature /°C 0 10 20 25 30 40 50 60 70 80 90 100 ε 87.88 84.01 80.23 78.41 76.63 73.23 69.93 66.79 63.80 60.87 58.13 55.51
One of the important properties of water is that it has a high dielectric constant. This constant shows its ability to make electrostatic bonds with other molecules, meaning it can eliminate the attraction of the opposite charges of the surrounding ions.
| temperature /°C | 0 | 10 | 20 | 25 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ε | 87.88 | 84.01 | 80.23 | 78.41 | 76.63 | 73.23 | 69.93 | 66.79 | 63.80 | 60.87 | 58.13 | 55.51 |
One of the important properties of water is that it has a high dielectric constant. This constant shows its ability to make electrostatic bonds with other molecules, meaning it can eliminate the attraction of the opposite charges of the surrounding ions.
Polarity, hydrogen bonding and inter-molecular structure
See also: Chemical polarity
An important feature of water is its polar nature. The structure has a bent molecular geometry for the two hydrogens from the oxygen vertex. The oxygen atom also has two lone pairs of electrons. One effect usually ascribed to the lone pairs is that the H–O–H gas phase bend angle is 104.48°,[37] which is smaller than the typical tetrahedral angle of 109.47°. The lone pair orbitals are more diffuse than thebond orbitals to the hydrogens; the increased repulsion of the lone pairs forces the O–H bonds closer to each other.[38]
Another effect of the electronic structure is that water is a polar molecule. There is a bond dipole moment pointing from each H to the O, making the oxygen partially negative and the hydrogen partially positive. In addition, the O also has nonbonded electrons in the direction opposite the hydrogen atoms. There is thus a large molecular dipole, pointing from a positive region between the two hydrogen atoms to the negative region of the oxygen atom. The charge differences cause water molecules to be attracted to each other (the relatively positive areas being attracted to the relatively negative areas) and to other polar molecules. This attraction contributes to hydrogen bonding, and explains many of the properties of water, such as solvent action.[39]
Although hydrogen bonding is a relatively weak attraction compared to the covalent bonds within the water molecule itself, it is responsible for a number of water's physical properties. One such property is its relatively high melting and boiling point temperatures; more energy is required to break the hydrogen bonds between water molecules. In contrast, hydrogen sulfide (H
2S), has much weaker hydrogen bonding due to sulfur's lower electronegativity. H
2S is a gas at room temperature, in spite of hydrogen sulfide having nearly two times the molar mass of water. The extra bonding between water molecules also gives liquid water a large specific heat capacity. This high heat capacity makes water a good heat storage medium (coolant) and heat shield.
See also: Chemical polarity
An important feature of water is its polar nature. The structure has a bent molecular geometry for the two hydrogens from the oxygen vertex. The oxygen atom also has two lone pairs of electrons. One effect usually ascribed to the lone pairs is that the H–O–H gas phase bend angle is 104.48°,[37] which is smaller than the typical tetrahedral angle of 109.47°. The lone pair orbitals are more diffuse than thebond orbitals to the hydrogens; the increased repulsion of the lone pairs forces the O–H bonds closer to each other.[38]
Another effect of the electronic structure is that water is a polar molecule. There is a bond dipole moment pointing from each H to the O, making the oxygen partially negative and the hydrogen partially positive. In addition, the O also has nonbonded electrons in the direction opposite the hydrogen atoms. There is thus a large molecular dipole, pointing from a positive region between the two hydrogen atoms to the negative region of the oxygen atom. The charge differences cause water molecules to be attracted to each other (the relatively positive areas being attracted to the relatively negative areas) and to other polar molecules. This attraction contributes to hydrogen bonding, and explains many of the properties of water, such as solvent action.[39]
Although hydrogen bonding is a relatively weak attraction compared to the covalent bonds within the water molecule itself, it is responsible for a number of water's physical properties. One such property is its relatively high melting and boiling point temperatures; more energy is required to break the hydrogen bonds between water molecules. In contrast, hydrogen sulfide (H
2S), has much weaker hydrogen bonding due to sulfur's lower electronegativity. H
2S is a gas at room temperature, in spite of hydrogen sulfide having nearly two times the molar mass of water. The extra bonding between water molecules also gives liquid water a large specific heat capacity. This high heat capacity makes water a good heat storage medium (coolant) and heat shield.
2S), has much weaker hydrogen bonding due to sulfur's lower electronegativity. H
2S is a gas at room temperature, in spite of hydrogen sulfide having nearly two times the molar mass of water. The extra bonding between water molecules also gives liquid water a large specific heat capacity. This high heat capacity makes water a good heat storage medium (coolant) and heat shield.
Proposed Structures
A single water molecule can participate in a maximum of four hydrogen bonds because it can accept two bonds using the lone pairs on oxygen and donate two hydrogen atoms. Other molecules like hydrogen fluoride, ammonia and methanol can also form hydrogen bonds. However they do not show anomalous thermodynamic, kinetic or structural properties like those observed in water. The answer to the apparent difference between water and other hydrogen bonding liquids lies in the fact that apart from water none of these examples participate in four hydrogen bonds; this is either due to an inability to donate or accept hydrogens or is due to steric effects in bulky residues. In water, intermolecular tetrahedral structures form due to the four hydrogen bonds gives rise to an open structure and a 3-dimensional bonding network, resulting in the anomalous decrease of density when cooled below 4 °C. This repeated, constantly reorganizing unit defines a three-dimensional network extending throughout the liquid. This view is based upon neutron scattering studies and computer simulations, and it makes sense in the light of the unambiguously tetrahedral arrangement of water molecules in ice structures.
However, there is an alternative theory for the structure of water. In 2004, a controversial paper published in science, from Stockholm University in Sweden, suggested that water molecules in liquid form, bind on average to not four but only two others; hence forming chains and rings. it was coined the term "string theory of water (not to be confused with string theory in physics). These observations were based upon X-ray absorption spectroscopy to probe the local environment of individual oxygen atoms. Water, the team now suggests, is a muddle of the two proposed structures. They say that it is a soup flecked with "ice bergs" each comprising 100 or so loosely connected molecules that are relatively open and hydrogen bonded. The soup is made of the string structure and the icebergs of the tetrahedral structure.[40]
A single water molecule can participate in a maximum of four hydrogen bonds because it can accept two bonds using the lone pairs on oxygen and donate two hydrogen atoms. Other molecules like hydrogen fluoride, ammonia and methanol can also form hydrogen bonds. However they do not show anomalous thermodynamic, kinetic or structural properties like those observed in water. The answer to the apparent difference between water and other hydrogen bonding liquids lies in the fact that apart from water none of these examples participate in four hydrogen bonds; this is either due to an inability to donate or accept hydrogens or is due to steric effects in bulky residues. In water, intermolecular tetrahedral structures form due to the four hydrogen bonds gives rise to an open structure and a 3-dimensional bonding network, resulting in the anomalous decrease of density when cooled below 4 °C. This repeated, constantly reorganizing unit defines a three-dimensional network extending throughout the liquid. This view is based upon neutron scattering studies and computer simulations, and it makes sense in the light of the unambiguously tetrahedral arrangement of water molecules in ice structures.
However, there is an alternative theory for the structure of water. In 2004, a controversial paper published in science, from Stockholm University in Sweden, suggested that water molecules in liquid form, bind on average to not four but only two others; hence forming chains and rings. it was coined the term "string theory of water (not to be confused with string theory in physics). These observations were based upon X-ray absorption spectroscopy to probe the local environment of individual oxygen atoms. Water, the team now suggests, is a muddle of the two proposed structures. They say that it is a soup flecked with "ice bergs" each comprising 100 or so loosely connected molecules that are relatively open and hydrogen bonded. The soup is made of the string structure and the icebergs of the tetrahedral structure.[40]
Cohesion and adhesion
Water molecules stay close to each other (cohesion), due to the collective action of hydrogen bonds between water molecules. These hydrogen bonds are constantly breaking, with new bonds being formed with different water molecules; but at any given time in a sample of liquid water, a large portion of the molecules are held together by such bonds.[41]
Water also has high adhesion properties because of its polar nature. On extremely clean/smooth glass the water may form a thin film because the molecular forces between glass and water molecules (adhesive forces) are stronger than the cohesive forces. In biological cells and organelles, water is in contact with membrane and protein surfaces that are hydrophilic; that is, surfaces that have a strong attraction to water. Irving Langmuir observed a strong repulsive force between hydrophilic surfaces. To dehydrate hydrophilic surfaces—to remove the strongly held layers of water of hydration—requires doing substantial work against these forces, called hydration forces. These forces are very large but decrease rapidly over a nanometer or less.[42] They are important in biology, particularly when cells are dehydrated by exposure to dry atmospheres or to extracellular freezing.[43]
Water molecules stay close to each other (cohesion), due to the collective action of hydrogen bonds between water molecules. These hydrogen bonds are constantly breaking, with new bonds being formed with different water molecules; but at any given time in a sample of liquid water, a large portion of the molecules are held together by such bonds.[41]
Water also has high adhesion properties because of its polar nature. On extremely clean/smooth glass the water may form a thin film because the molecular forces between glass and water molecules (adhesive forces) are stronger than the cohesive forces. In biological cells and organelles, water is in contact with membrane and protein surfaces that are hydrophilic; that is, surfaces that have a strong attraction to water. Irving Langmuir observed a strong repulsive force between hydrophilic surfaces. To dehydrate hydrophilic surfaces—to remove the strongly held layers of water of hydration—requires doing substantial work against these forces, called hydration forces. These forces are very large but decrease rapidly over a nanometer or less.[42] They are important in biology, particularly when cells are dehydrated by exposure to dry atmospheres or to extracellular freezing.[43]
Surface tension
Main article: Surface tension
Water has a high surface tension of 72.8 mN/m at room temperature, caused by the strong cohesion between water molecules, the highest of the common non-ionic, non-metallic liquids. This can be seen when small quantities of water are placed onto a sorption-free (non-adsorbent and non-absorbent) surface, such aspolyethylene or Teflon, and the water stays together as drops. Just as significantly, air trapped in surface disturbances forms bubbles, which sometimes last long enough to transfer gas molecules to the water.[citation needed]
Another surface tension effect is capillary waves, which are the surface ripples that form around the impacts of drops on water surfaces, and sometimes occur with strong subsurface currents flowing to the water surface. The apparent elasticity caused by surface tension drives the waves.
Main article: Surface tension
Water has a high surface tension of 72.8 mN/m at room temperature, caused by the strong cohesion between water molecules, the highest of the common non-ionic, non-metallic liquids. This can be seen when small quantities of water are placed onto a sorption-free (non-adsorbent and non-absorbent) surface, such aspolyethylene or Teflon, and the water stays together as drops. Just as significantly, air trapped in surface disturbances forms bubbles, which sometimes last long enough to transfer gas molecules to the water.[citation needed]
Another surface tension effect is capillary waves, which are the surface ripples that form around the impacts of drops on water surfaces, and sometimes occur with strong subsurface currents flowing to the water surface. The apparent elasticity caused by surface tension drives the waves.
Capillary action
Main article: Capillary action
Due to an interplay of the forces of adhesion and surface tension, water exhibits capillary action whereby water rises into a narrow tube against the force of gravity. Water adheres to the inside wall of the tube and surface tension tends to straighten the surface causing a surface rise and more water is pulled up through cohesion. The process continues as the water flows up the tube until there is enough water such that gravity balances the adhesive force.
Surface tension and capillary action are important in biology. For example, when water is carried through xylem up stems in plants, the strong intermolecular attractions (cohesion) hold the water column together and adhesive properties maintain the water attachment to the xylem and prevent tension rupture caused by transpiration pull.
Main article: Capillary action
Due to an interplay of the forces of adhesion and surface tension, water exhibits capillary action whereby water rises into a narrow tube against the force of gravity. Water adheres to the inside wall of the tube and surface tension tends to straighten the surface causing a surface rise and more water is pulled up through cohesion. The process continues as the water flows up the tube until there is enough water such that gravity balances the adhesive force.
Surface tension and capillary action are important in biology. For example, when water is carried through xylem up stems in plants, the strong intermolecular attractions (cohesion) hold the water column together and adhesive properties maintain the water attachment to the xylem and prevent tension rupture caused by transpiration pull.
Water as a solvent
Main article: Aqueous solution
Water is also a good solvent, due to its polarity. Substances that will mix well and dissolve in water (e.g. salts) are known as hydrophilic ("water-loving") substances, while those that do not mix well with water (e.g. fats and oils), are known as hydrophobic ("water-fearing") substances. The ability of a substance to dissolve in water is determined by whether or not the substance can match or better the strong attractive forces that water molecules generate between other water molecules. If a substance has properties that do not allow it to overcome these strong intermolecular forces, the molecules are "pushed out" from the water, and do not dissolve. Contrary to the common misconception, water and hydrophobic substances do not "repel", and the hydration of a hydrophobic surface is energetically, but not entropically, favorable.
When an ionic or polar compound enters water, it is surrounded by water molecules (Hydration). The relatively small size of water molecules (~ 3 Angstroms) allows many water molecules to surround one molecule of solute. The partially negative dipole ends of the water are attracted to positively charged components of the solute, and vice versa for the positive dipole ends.
In general, ionic and polar substances such as acids, alcohols, and salts are relatively soluble in water, and non-polar substances such as fats and oils are not. Non-polar molecules stay together in water because it is energetically more favorable for the water molecules to hydrogen bond to each other than to engage in van der Waals interactions with non-polar molecules.
An example of an ionic solute is table salt; the sodium chloride, NaCl, separates into Na+ cations and Cl− anions, each being surrounded by water molecules. The ions are then easily transported away from their crystalline lattice into solution. An example of a nonionic solute is table sugar. The water dipoles make hydrogen bonds with the polar regions of the sugar molecule (OH groups) and allow it to be carried away into solution.
Main article: Aqueous solution
Water is also a good solvent, due to its polarity. Substances that will mix well and dissolve in water (e.g. salts) are known as hydrophilic ("water-loving") substances, while those that do not mix well with water (e.g. fats and oils), are known as hydrophobic ("water-fearing") substances. The ability of a substance to dissolve in water is determined by whether or not the substance can match or better the strong attractive forces that water molecules generate between other water molecules. If a substance has properties that do not allow it to overcome these strong intermolecular forces, the molecules are "pushed out" from the water, and do not dissolve. Contrary to the common misconception, water and hydrophobic substances do not "repel", and the hydration of a hydrophobic surface is energetically, but not entropically, favorable.
When an ionic or polar compound enters water, it is surrounded by water molecules (Hydration). The relatively small size of water molecules (~ 3 Angstroms) allows many water molecules to surround one molecule of solute. The partially negative dipole ends of the water are attracted to positively charged components of the solute, and vice versa for the positive dipole ends.
In general, ionic and polar substances such as acids, alcohols, and salts are relatively soluble in water, and non-polar substances such as fats and oils are not. Non-polar molecules stay together in water because it is energetically more favorable for the water molecules to hydrogen bond to each other than to engage in van der Waals interactions with non-polar molecules.
An example of an ionic solute is table salt; the sodium chloride, NaCl, separates into Na+ cations and Cl− anions, each being surrounded by water molecules. The ions are then easily transported away from their crystalline lattice into solution. An example of a nonionic solute is table sugar. The water dipoles make hydrogen bonds with the polar regions of the sugar molecule (OH groups) and allow it to be carried away into solution.
Water in acid-base reactions
Chemically, water is amphoteric: it has the rare ability to act as either an acid or a base in chemical reactions. According to the Brønsted-Lowry definition, an acid is defined as a species which donates a proton (a H+ ion) in a reaction, and a base as one which receives a proton. When reacting with a stronger acid, water acts as a base; when reacting with a stronger base, it acts as an acid. For instance, water receives an H+ ion from HCl when hydrochloric acid is formed:
- HCl (acid) + H
2O (base) ⇌ H
3O+ + Cl−
- NH
3 (base) + H
2O (acid) ⇌ NH+
4 + OH−
Because the oxygen atom in water has two lone pairs, water often acts as a Lewis base, or electron pair donor, in reactions with Lewis acids, although it can also react with Lewis bases, forming hydrogen bonds between the electron pair donors and the hydrogen atoms of water. HSAB theory describes water as both a weak hard acid and a weak hard base, meaning that it reacts preferentially with other hard species:
- H+ (Lewis acid) + H
2O (Lewis base) → H
3O+
- Fe3+ (Lewis acid) + H
2O (Lewis base) → Fe(H
2O)3+
6
- Cl− (Lewis base) + H
2O (Lewis acid) → Cl(H
2O)−
6
When a salt of a weak acid or of a weak base is dissolved in water, water can partially hydrolyze the salt, producing the corresponding base or acid, which gives aqueous solutions of soap and baking soda their basic pH:
- Na
2CO
3 + H
2O ⇌ NaOH + NaHCO
3
Chemically, water is amphoteric: it has the rare ability to act as either an acid or a base in chemical reactions. According to the Brønsted-Lowry definition, an acid is defined as a species which donates a proton (a H+ ion) in a reaction, and a base as one which receives a proton. When reacting with a stronger acid, water acts as a base; when reacting with a stronger base, it acts as an acid. For instance, water receives an H+ ion from HCl when hydrochloric acid is formed:
- HCl (acid) + H
2O (base) ⇌ H
3O+ + Cl−
- NH
3 (base) + H
2O (acid) ⇌ NH+
4 + OH−
Because the oxygen atom in water has two lone pairs, water often acts as a Lewis base, or electron pair donor, in reactions with Lewis acids, although it can also react with Lewis bases, forming hydrogen bonds between the electron pair donors and the hydrogen atoms of water. HSAB theory describes water as both a weak hard acid and a weak hard base, meaning that it reacts preferentially with other hard species:
- H+ (Lewis acid) + H
2O (Lewis base) → H
3O+
- Fe3+ (Lewis acid) + H
2O (Lewis base) → Fe(H
2O)3+
6
- Cl− (Lewis base) + H
2O (Lewis acid) → Cl(H
2O)−
6
When a salt of a weak acid or of a weak base is dissolved in water, water can partially hydrolyze the salt, producing the corresponding base or acid, which gives aqueous solutions of soap and baking soda their basic pH:
- Na
2CO
3 + H
2O ⇌ NaOH + NaHCO
3
Ligand chemistry
Water's Lewis base character makes it a common ligand in transition metal complexes, examples of which range from solvated ions, such as Fe(H
2O)3+
6, to perrhenic acid, which contains two water molecules coordinated to a rhenium atom, to various solid hydrates, such as CoCl
2·6H
2O. Water is typically a monodentate ligand–it forms only one bond with the central atom.
Water's Lewis base character makes it a common ligand in transition metal complexes, examples of which range from solvated ions, such as Fe(H
2O)3+
6, to perrhenic acid, which contains two water molecules coordinated to a rhenium atom, to various solid hydrates, such as CoCl
2·6H
2O. Water is typically a monodentate ligand–it forms only one bond with the central atom.
2O)3+
6, to perrhenic acid, which contains two water molecules coordinated to a rhenium atom, to various solid hydrates, such as CoCl
2·6H
2O. Water is typically a monodentate ligand–it forms only one bond with the central atom.
Organic chemistry
As a hard base, water reacts readily with organic carbocations, for example in hydration reaction, in which a hydroxyl group (OH−) and an acidic proton are added to the two carbon atoms bonded together in the carbon-carbon double bond, resulting in an alcohol. When addition of water to an organic molecule cleaves the molecule in two, hydrolysisis said to occur. Notable examples of hydrolysis are saponification of fats and digestion of proteins and polysaccharides. Water can also be a leaving group in SN2 substitutionand E2 elimination reactions, the latter is then known as dehydration reaction.
As a hard base, water reacts readily with organic carbocations, for example in hydration reaction, in which a hydroxyl group (OH−) and an acidic proton are added to the two carbon atoms bonded together in the carbon-carbon double bond, resulting in an alcohol. When addition of water to an organic molecule cleaves the molecule in two, hydrolysisis said to occur. Notable examples of hydrolysis are saponification of fats and digestion of proteins and polysaccharides. Water can also be a leaving group in SN2 substitutionand E2 elimination reactions, the latter is then known as dehydration reaction.
Acidity in nature
Pure water has the concentration of hydroxide ions (OH−) equal to that of the hydronium (H
3O+) or hydrogen (H+) ions, which gives pH of 7 at 298 K. In practice, pure water is very difficult to produce. Water left exposed to air for any length of time will dissolve carbon dioxide, forming a dilute solution of carbonic acid, with a limiting pH of about 5.7. As cloud droplets form in the atmosphere and as raindrops fall through the air minor amounts of CO
2 are absorbed, and thus most rain is slightly acidic. If high amounts of nitrogenand sulfur oxides are present in the air, they too will dissolve into the cloud and rain drops, producing acid rain.
Pure water has the concentration of hydroxide ions (OH−) equal to that of the hydronium (H
3O+) or hydrogen (H+) ions, which gives pH of 7 at 298 K. In practice, pure water is very difficult to produce. Water left exposed to air for any length of time will dissolve carbon dioxide, forming a dilute solution of carbonic acid, with a limiting pH of about 5.7. As cloud droplets form in the atmosphere and as raindrops fall through the air minor amounts of CO
2 are absorbed, and thus most rain is slightly acidic. If high amounts of nitrogenand sulfur oxides are present in the air, they too will dissolve into the cloud and rain drops, producing acid rain.
3O+) or hydrogen (H+) ions, which gives pH of 7 at 298 K. In practice, pure water is very difficult to produce. Water left exposed to air for any length of time will dissolve carbon dioxide, forming a dilute solution of carbonic acid, with a limiting pH of about 5.7. As cloud droplets form in the atmosphere and as raindrops fall through the air minor amounts of CO
2 are absorbed, and thus most rain is slightly acidic. If high amounts of nitrogenand sulfur oxides are present in the air, they too will dissolve into the cloud and rain drops, producing acid rain.
Water in redox reactions
Water contains hydrogen in oxidation state +1 and oxygen in oxidation state −2. Because of that, water oxidizes chemicals with reduction potential below the potential of H+/H
2, such as hydrides, alkali and alkaline earth metals, etc. Some other reactive metals, such as aluminum and beryllium, are oxidized by water as well, but their oxides are not soluble, and the reaction stops because of passivation. Note, however, that rusting of iron is a reaction between iron and oxygen, dissolved in water, not between iron and water.
- 2 Na + 2 H
2O → 2 NaOH + H
2
Water itself can be oxidized, emitting oxygen gas, but very few oxidants react with water even if their reduction potential is greater than the potential of O
2/H
2O. Almost all such reactions require a catalyst.[44]
- 4 AgF
2 + 2 H
2O → 4 AgF + 4 HF + O
2
Water contains hydrogen in oxidation state +1 and oxygen in oxidation state −2. Because of that, water oxidizes chemicals with reduction potential below the potential of H+/H
2, such as hydrides, alkali and alkaline earth metals, etc. Some other reactive metals, such as aluminum and beryllium, are oxidized by water as well, but their oxides are not soluble, and the reaction stops because of passivation. Note, however, that rusting of iron is a reaction between iron and oxygen, dissolved in water, not between iron and water.
2, such as hydrides, alkali and alkaline earth metals, etc. Some other reactive metals, such as aluminum and beryllium, are oxidized by water as well, but their oxides are not soluble, and the reaction stops because of passivation. Note, however, that rusting of iron is a reaction between iron and oxygen, dissolved in water, not between iron and water.
- 2 Na + 2 H
2O → 2 NaOH + H
2
Water itself can be oxidized, emitting oxygen gas, but very few oxidants react with water even if their reduction potential is greater than the potential of O
2/H
2O. Almost all such reactions require a catalyst.[44]
2/H
2O. Almost all such reactions require a catalyst.[44]
- 4 AgF
2 + 2 H
2O → 4 AgF + 4 HF + O
2
Geochemistry
Action of water on rock over long periods of time typically leads to weathering and water erosion, physical processes that convert solid rocks and minerals into soil and sediment, but under some conditions chemical reactions with water occur as well, resulting in metasomatism or mineral hydration, a type of chemical alteration of a rock which producesclay minerals in nature and also occurs when Portland cement hardens.
Water ice can form clathrate compounds, known as clathrate hydrates, with a variety of small molecules that can be embedded in its spacious crystal lattice. The most notable of these is methane clathrate, 4 CH
4·23 H
2O, naturally found in large quantities on the ocean floor.
Action of water on rock over long periods of time typically leads to weathering and water erosion, physical processes that convert solid rocks and minerals into soil and sediment, but under some conditions chemical reactions with water occur as well, resulting in metasomatism or mineral hydration, a type of chemical alteration of a rock which producesclay minerals in nature and also occurs when Portland cement hardens.
Water ice can form clathrate compounds, known as clathrate hydrates, with a variety of small molecules that can be embedded in its spacious crystal lattice. The most notable of these is methane clathrate, 4 CH
4·23 H
2O, naturally found in large quantities on the ocean floor.
4·23 H
2O, naturally found in large quantities on the ocean floor.
Transparency
Main article: Electromagnetic absorption by water
Water is relatively transparent to visible light, near ultraviolet light, and far-red light, but it absorbs most ultraviolet light, infrared light, and microwaves. Most photoreceptors andphotosynthetic pigments utilize the portion of the light spectrum that is transmitted well through water. Microwave ovens take advantage of water's opacity to microwave radiation to heat the water inside of foods. The very weak onset of absorption in the red end of the visible spectrum lends water its intrinsic blue hue (see Color of water).
Main article: Electromagnetic absorption by water
Water is relatively transparent to visible light, near ultraviolet light, and far-red light, but it absorbs most ultraviolet light, infrared light, and microwaves. Most photoreceptors andphotosynthetic pigments utilize the portion of the light spectrum that is transmitted well through water. Microwave ovens take advantage of water's opacity to microwave radiation to heat the water inside of foods. The very weak onset of absorption in the red end of the visible spectrum lends water its intrinsic blue hue (see Color of water).
Heavy water and isotopologues
Several isotopes of both hydrogen and oxygen exist, giving rise to several known isotopologues of water.
Hydrogen occurs naturally in three isotopes. The most common isotope, 1H, which accounts for more than 99.98% of hydrogen in water, consists of only a single proton in its nucleus. A second, stable isotope, deuterium (chemical symbol D or 2H), has an additional neutron. Deuterium oxide, D
2O, is also known as heavy water because of its higher density. It is used in nuclear reactors as a neutron moderator. The third isotope, tritium (Chemical symbol T or 3H) has 1 proton and 2 neutrons, and is radioactive, decaying with a half-life of 4500 days. THO exists in nature only in minute quantities, being produced primarily via cosmic ray-induced nuclear reactions in the atmosphere. Water with one deuterium atom HDO occurs naturally in ordinary water in low concentrations (~0.03%) and D
2O in far lower amounts (0.000003%).
The most notable physical differences between H
2O and D
2O, other than the simple difference in specific mass, involve properties that are affected by hydrogen bonding, such as freezing and boiling, and other kinetic effects. The difference in boiling points allows the isotopologues to be separated. The self-diffusion coefficient of H
2O at 25 °C is 23% higher than the value of D
2O.[45]
Consumption of pure isolated D
2O may affect biochemical processes – ingestion of large amounts impairs kidney and central nervous system function. Small quantities can be consumed without any ill-effects, and very large amounts of heavy water must be consumed for any toxicity to become apparent.
Oxygen also has three stable isotopes, with 16O present in 99.76%, 17O in 0.04%, and 18O in 0.2% of water molecules.[46]
Several isotopes of both hydrogen and oxygen exist, giving rise to several known isotopologues of water.
Hydrogen occurs naturally in three isotopes. The most common isotope, 1H, which accounts for more than 99.98% of hydrogen in water, consists of only a single proton in its nucleus. A second, stable isotope, deuterium (chemical symbol D or 2H), has an additional neutron. Deuterium oxide, D
2O, is also known as heavy water because of its higher density. It is used in nuclear reactors as a neutron moderator. The third isotope, tritium (Chemical symbol T or 3H) has 1 proton and 2 neutrons, and is radioactive, decaying with a half-life of 4500 days. THO exists in nature only in minute quantities, being produced primarily via cosmic ray-induced nuclear reactions in the atmosphere. Water with one deuterium atom HDO occurs naturally in ordinary water in low concentrations (~0.03%) and D
2O in far lower amounts (0.000003%).
2O, is also known as heavy water because of its higher density. It is used in nuclear reactors as a neutron moderator. The third isotope, tritium (Chemical symbol T or 3H) has 1 proton and 2 neutrons, and is radioactive, decaying with a half-life of 4500 days. THO exists in nature only in minute quantities, being produced primarily via cosmic ray-induced nuclear reactions in the atmosphere. Water with one deuterium atom HDO occurs naturally in ordinary water in low concentrations (~0.03%) and D
2O in far lower amounts (0.000003%).
The most notable physical differences between H
2O and D
2O, other than the simple difference in specific mass, involve properties that are affected by hydrogen bonding, such as freezing and boiling, and other kinetic effects. The difference in boiling points allows the isotopologues to be separated. The self-diffusion coefficient of H
2O at 25 °C is 23% higher than the value of D
2O.[45]
2O and D
2O, other than the simple difference in specific mass, involve properties that are affected by hydrogen bonding, such as freezing and boiling, and other kinetic effects. The difference in boiling points allows the isotopologues to be separated. The self-diffusion coefficient of H
2O at 25 °C is 23% higher than the value of D
2O.[45]
Consumption of pure isolated D
2O may affect biochemical processes – ingestion of large amounts impairs kidney and central nervous system function. Small quantities can be consumed without any ill-effects, and very large amounts of heavy water must be consumed for any toxicity to become apparent.
2O may affect biochemical processes – ingestion of large amounts impairs kidney and central nervous system function. Small quantities can be consumed without any ill-effects, and very large amounts of heavy water must be consumed for any toxicity to become apparent.
Oxygen also has three stable isotopes, with 16O present in 99.76%, 17O in 0.04%, and 18O in 0.2% of water molecules.[46]
History
The first decomposition of water into hydrogen and oxygen, by electrolysis, was done in 1800 by English chemist William Nicholson. In 1805, Joseph Louis Gay-Lussac andAlexander von Humboldt showed that water is composed of two parts hydrogen and one part oxygen.
The properties of water have historically been used to define various temperature scales. Notably, the Kelvin, Celsius, Rankine, and Fahrenheit scales were, or currently are, defined by the freezing and boiling points of water. The less common scales of Delisle, Newton, Réaumur and Rømer were defined similarly. The triple point of water is a more commonly used standard point today.[48]
The first decomposition of water into hydrogen and oxygen, by electrolysis, was done in 1800 by English chemist William Nicholson. In 1805, Joseph Louis Gay-Lussac andAlexander von Humboldt showed that water is composed of two parts hydrogen and one part oxygen.
The properties of water have historically been used to define various temperature scales. Notably, the Kelvin, Celsius, Rankine, and Fahrenheit scales were, or currently are, defined by the freezing and boiling points of water. The less common scales of Delisle, Newton, Réaumur and Rømer were defined similarly. The triple point of water is a more commonly used standard point today.[48]
Systematic naming
The accepted IUPAC name of water is oxidane[49] or simply water, or its equivalent in different languages, although there are other systematic names which can be used to describe the molecule.
The simplest systematic name of water is hydrogen oxide. This is analogous to related compounds such as hydrogen peroxide, hydrogen sulfide, and deuterium oxide (heavy water). Another systematic name, oxidane, is accepted by IUPAC as a parent name for the systematic naming of oxygen-based substituent groups,[50] although even these commonly have other recommended names. For example, the name hydroxyl is recommended over oxidanyl for the –OH group. The name oxane is explicitly mentioned by the IUPAC as being unsuitable for this purpose, since it is already the name of a cyclic ether also known as tetrahydropyran.
The polarized form of the water molecule, H+OH−, is also called hydron hydroxide by IUPAC nomenclature.[51]
In keeping with the basic rules of chemical nomenclature, water would have a systematic name of dihydrogen monoxide,[52] but this is not among the names published by theInternational Union of Pure and Applied Chemistry.[53] It is a rarely used name of water, and mostly used in various hoaxes or spoofs that call for this "lethal chemical" to be banned, such as in the dihydrogen monoxide hoax.
Other systematic names for water include hydroxic acid, hydroxylic acid, and hydrogen hydroxide, using acid and base names.[h] None of these exotic names are used widely.
The accepted IUPAC name of water is oxidane[49] or simply water, or its equivalent in different languages, although there are other systematic names which can be used to describe the molecule.
The simplest systematic name of water is hydrogen oxide. This is analogous to related compounds such as hydrogen peroxide, hydrogen sulfide, and deuterium oxide (heavy water). Another systematic name, oxidane, is accepted by IUPAC as a parent name for the systematic naming of oxygen-based substituent groups,[50] although even these commonly have other recommended names. For example, the name hydroxyl is recommended over oxidanyl for the –OH group. The name oxane is explicitly mentioned by the IUPAC as being unsuitable for this purpose, since it is already the name of a cyclic ether also known as tetrahydropyran.
The polarized form of the water molecule, H+OH−, is also called hydron hydroxide by IUPAC nomenclature.[51]
In keeping with the basic rules of chemical nomenclature, water would have a systematic name of dihydrogen monoxide,[52] but this is not among the names published by theInternational Union of Pure and Applied Chemistry.[53] It is a rarely used name of water, and mostly used in various hoaxes or spoofs that call for this "lethal chemical" to be banned, such as in the dihydrogen monoxide hoax.
Other systematic names for water include hydroxic acid, hydroxylic acid, and hydrogen hydroxide, using acid and base names.[h] None of these exotic names are used widely.




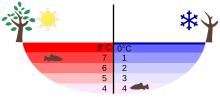

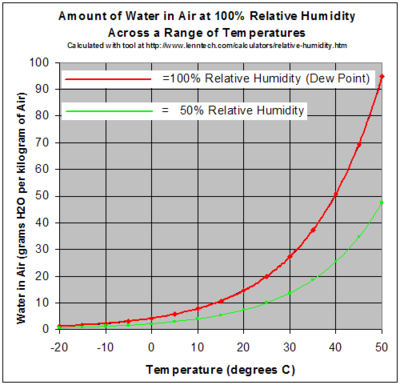








No comments:
Post a Comment