Black Wall Street[no edit]
During the oil boom of the 1910s, the area of northeast Oklahoma around Tulsa flourished, including the Greenwood neighborhood, which came to be known as "the Negro Wall Street" (now commonly referred to as "the Black Wall Street").[3] The area was home to several prominent black businessmen. Greenwood boasted a variety of thriving businesses that were very successful up until the Tulsa Race Riot. Not only did black Americans want to contribute to the success of their own shops, but there were also racial segregation laws that prevented them from shopping anywhere other than Greenwood.[4] Following the riots, the area was rebuilt and thrived until the 1960s when desegregation allowed blacks to shop in areas from which they were previously restricted.
Detroit Avenue, along the edge of Standpipe Hill, contained a number of expensive houses belonging to doctors, lawyers and business owners. The buildings on Greenwood Avenue housed the offices of almost all of Tulsa’s black lawyers, realtors, doctors, and other professionals.[5] In Tulsa at the time of the riot, there were fifteen well-known black American physicians, one of whom, Dr. A.C. Jackson, was considered the "most able Negro surgeon in America" by one of the Mayo brothers.[6] Dr. Jackson was shot to death as he left his house during the unrest.[2] Greenwood published two newspapers, the Tulsa Star and the Oklahoma Sun, which covered not only Tulsa, but also state and national news and elections. The buildings that housed the newspapers were destroyed during the destruction of Greenwood.[2]
Greenwood was a very religiously active community. At the time of the racial violence there were more than a dozen black American churches and many Christian youth organizations and religious societies.[citation needed]
In northeastern Oklahoma, as elsewhere in America, the prosperity of minorities emerged amidst racial and political tension. The Ku Klux Klan made its first major appearance in Oklahoma shortly before one of the worst race riots in history.[7] It is estimated that there were about 3,200 members of the Klan in Tulsa in 1921.[no citation needed]
Energy density
From Wikipedia, the free encyclopedia
This article is about energy per unit volume. For energy per unit mass or energy density of foods, see specific energy.
| Energy density | |
|---|---|
| SI unit | J/m3 |
| In SI base units | kg·m-1s-2 |
Derivations from
other quantities | U = E/V |
Energy density is the amount of energy stored in a given system or region of space per unit volume or mass, though the latter is more accurately termed specific energy. Often only the useful or extractable energy is measured, which is to say that chemically inaccessible energy such as rest mass energy is ignored.[1] In cosmological and other general relativistic contexts, however, the energy densities considered are those that correspond to the elements of the stress–energy tensor and therefore do include mass energy as well as energy densities associated with the pressures described in the next paragraph.
Energy per unit volume has the same physical units as pressure, and in many circumstances is a synonym: for example, the energy density of a magnetic field may be expressed as (and behaves as) a physical pressure, and the energy required to compress a compressed gas a little more may be determined by multiplying the difference between the gas pressure and the external pressure by the change in volume. In short, pressure is a measure of the enthalpy per unit volume of a system. A pressure gradient has a potential to perform work on the surroundings by converting enthalpy until equilibrium is reached.
Contents
[hide]Introduction to energy density[edit]
There are many different types of energy stored in materials, and it takes a particular type of reaction to release each type of energy. In order of the typical magnitude of the energy released, these types of reactions are: nuclear, chemical, electrochemical, and electrical.
Chemical reactions are used by animals to derive energy from food, and by automobiles to derive energy from gasoline. Electrochemical reactions are used by most mobile devices such as laptop computers and mobile phones to release the energy from batteries.
Energy densities of common energy storage materials[edit]
| This section does not cite any sources. (October 2013) |
The following is a list of the thermal energy densities of commonly used or well-known energy storage materials; it doesn't include uncommon or experimental materials. Note that this list does not consider the mass of reactants commonly available such as the oxygen required for combustion or the energy efficiency in use.
The following unit conversions may be helpful when considering the data in the table: 1 MJ ≈ 0.28 kWh ≈ 0.37 HPh.
| Storage material | Energy type | Specific energy (MJ/kg) | Energy density (MJ/L) | Direct uses |
|---|---|---|---|---|
| Uranium (in breeder) | Nuclear fission | 80,620,000[2] | 1,539,842,000 | Electric power plants (nuclear reactors), industrial process heat (to drive chemical reactions, water desalination, etc.) |
| Thorium (in breeder) | Nuclear fission | 79,420,000[2] | 929,214,000 | Electric power plants (nuclear reactors), industrial process heat |
| Plutonium | Nuclear decay | 2,239,000 | ? | Thermal-Electric Generator (Space) |
| Tritium | Nuclear decay | 583,529 | ? | Electric power plants (nuclear reactors), industrial process heat |
| Hydrogen (compressed at 700 bar) | Chemical | 142 | 5.6 | Rocket engines, automotive engines, grid storage & conversion |
| Methane or natural gas | Chemical | 55.5 | 0.0364 | Cooking, home heating, automotive engines |
| Diesel / Fuel oil | Chemical | 48 | 35.8 | Automotive engines, power plants[3] |
| LPG (including Propane /Butane) | Chemical | 46.4 | 26 | Cooking, home heating, automotive engines, lighter fluid |
| Jet fuel (Kerosene) | Chemical | 46[citation needed] | 37.4 | Aircraft |
| Gasoline (petrol) | Chemical | 46.4 | 34.2 | Automotive engines, power plants[4] |
| Fat (animal/vegetable) | Chemical | 37 | 34 | Human/animal nutrition |
| Dimethyl ether (DME) | Chemical | 28.8[5] | 19.3 | Diesel cycle, Gas turbine, LPG applications |
| Ethanol fuel (E100) | Chemical | 26.4 | 20.9 | Flex-fuel, racing, stoves, lighting |
| Coal, anthracite | Chemical | 26-33 | 34-43 | Electric power plants, home heating |
| Coal, bituminous | Chemical | 24-35 | 26-49 | Electric power plants, home heating |
| Methanol fuel (M100) | Chemical | 19.7 | 15.6 | Racing, model engines, safety |
| Carbohydrates(including sugars) | Chemical | 17 | Human/animal nutrition | |
| Protein | Chemical | 16.8 | Human/animal nutrition | |
| Wood | Chemical | 16.2[citation needed] | 13 | Heating, outdoor cooking |
| TNT | Chemical | 4.6 | Explosives | |
| Gunpowder | Chemical | 3[citation needed] | Explosives | |
| Lithium battery (non-rechargeable) | Electrochemical | 1.8 | 4.32 | Portable electronic devices, flashlights |
| Lithium-ion battery | Electrochemical | 0.36[6]–0.875[7] | 0.9–2.63 | Laptop computers, mobile devices, electric vehicles |
| Alkaline battery | Electrochemical | 0.5[8] | 1.3[8] | Portable electronic devices, flashlights |
| Nickel-metal hydride battery | Electrochemical | 0.288 | 0.504–1.08 | Portable electronic devices, flashlights |
| Lead-acid battery | Electrochemical | 0.17 | 0.56 | Automotive engine ignition |
| Supercapacitor (EDLC) | Electrical (electrostatic) | 0.01-0.036[9][10][11][12][13][14] | 0.06-0.05[9][10][11][12][13][14] | Electronic circuits |
| Supercapacitor (Pseudo) | Electrochemical | 0.031[15] | 0.046[15] | Electronic circuits |
| Electrostatic capacitor | Electrical (electrostatic) | 0.00001-0.0002[16] | 0.00001-0.001[16][17][18] | Electronic circuits |
| Storage device | Energy type | Energy content (MJ) | Typical mass | Specific energy (MJ/kg) | W × H × D (mm) | Uses |
|---|---|---|---|---|---|---|
| Automotive lead-acid battery | Electrochemical | 2.6 | 15 kg | 0.17 | 230 × 180 × 185 | Automotive starter motor and accessories |
| Alkaline AA battery | Electrochemical | 0.0154 | 23 g | 0.669 | 14.5 × 50.5 × 14.5 | Portable electronic equipment, flashlights |
| Lithium-ion battery [19] | Electrochemical | 0.0129 | 20 g | 0.645 | 54.2 × 33.8 × 5.8 | Mobile phones |
Energy density in energy storage and in fuel[edit]
In energy storage applications the energy density relates the mass of an energy store to the volume of the storage facility, e.g. the fuel tank. The higher the energy density of the fuel, the more energy may be stored or transported for the same amount of volume. The energy density of a fuel per unit mass is called the specific energy of that fuel. In general an engine using that fuel will generate less kinetic energy due to inefficienciesand thermodynamic considerations—hence the specific fuel consumption of an engine will always be greater than its rate of production of the kinetic energy of motion.
The greatest energy source by far is mass itself. This energy, E = mc2, where m = ρV, ρ is the mass per unit volume, V is the volume of the mass itself and c is the speed of light. This energy, however, can be released only by the processes of nuclear fission (.1%), nuclear fusion (1%),[citation needed] or the annihilation of some or all of the matter in the volume V by matter-antimatter collisions (100%). Nuclear reactions cannot be realized by chemical reactions such as combustion. Although greater matter densities can be achieved, the density of a neutron star would approximate the most dense system capable of matter-antimatter annihilation possible. A black hole, although denser than a neutron star, does not have an equivalent anti-particle form, but would offer the same 100% conversion rate of mass to energy in the form of Hawking radiation. In the case of relatively small black holes (smaller than astronomical objects) the power output would be tremendous.
The highest density sources of energy aside from antimatter are fusion and fission. Fusion includes energy from the sun which will be available for billions of years (in the form of sunlight) but so far (2011), sustained fusion power production continues to be elusive. Power from fission of uranium and thorium in nuclear power plants will be available for a many decades or even centuries because of the plentiful supply of the elements on earth,[20] though the full potential of this source can only be realised through breeder reactors, which are, apart from the BN-600 reactor, not yet used commercially.[21] Coal, gas, and petroleum are the current primary energy sources in the U.S.[22] but have a much lower energy density. Burning local biomass fuels supplies household energy needs (cooking fires, oil lamps, etc.) worldwide.
Energy density (how much energy you can carry) does not tell you about energy conversion efficiency (net output per input) or embodied energy (what the energy output costs to provide, as harvesting, refining, distributing, and dealing with pollution all use energy). Like any process occurring on a large scale, intensive energy use impacts the world. For example, climate change, nuclear waste storage, and deforestation may be some of the consequences of supplying our growing energy demands from hydrocarbon fuels, nuclear fission, or biomass.
No single energy storage method boasts the best in specific power, specific energy, and energy density. Peukert's Law describes how the amount of useful energy that can be obtained (for a lead-acid cell) depends on how quickly we pull it out. To maximize both specific energy and energy density, one can compute the specific energy density of a substance by multiplying the two values together, where the higher the number, the better the substance is at storing energy efficiently.
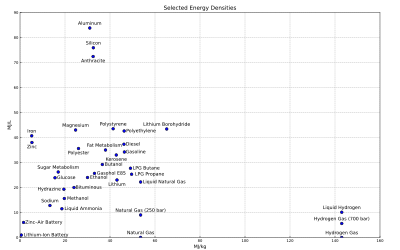
Gravimetric and volumetric energy density of some fuels and storage technologies (modified from the Gasoline article):
- Note: Some values may not be precise because of isomers or other irregularities. See Heating value for a comprehensive table of specific energies of important fuels.
- Note: Also it is important to realise that generally the density values for chemical fuels do not include the weight of oxygen required for combustion. This is typically two oxygen atoms per carbon atom, and one per two hydrogen atoms. The atomic weight of carbon and oxygen are similar, while hydrogen is much lighter than oxygen. Figures are presented this way for those fuels where in practice air would only be drawn in locally to the burner. This explains the apparently lower energy density of materials that already include their own oxidiser (such as gunpowder and TNT), where the mass of the oxidiser in effect adds dead weight, and absorbs some of the energy of combustion to dissociate and liberate oxygen to continue the reaction. This also explains some apparent anomalies, such as the energy density of a sandwich appearing to be higher than that of a stick of dynamite.
| This section may require cleanup to meet Wikipedia's quality standards. (October 2008) |
Energy densities ignoring external components[edit]
This table lists energy densities of systems that require external components, such as oxidisers or a heat sink or source. These figures do not take into account the mass and volume of the required components as they are assumed to be freely available and present in the atmosphere. Such systems cannot be compared with self-contained systems. These values may not be computed at the same reference conditions. Most of them seem to be higher heating value (HHV).
| Storage type | Specific energy (MJ/kg) | Energy density (MJ/L) | Peak recovery efficiency % | Practical recovery efficiency % |
|---|---|---|---|---|
| Antimatter | 9×1010 = 1*c^2 (assuming c is in m/s) | Density would depend on the form the antimatter takes | 100 | |
| Hydrogen, liquid[23] | 141.86 | 8.491 | ||
| Hydrogen, at 690 bar and 15°C[23] | 141.86 | 4.5 | ||
| Hydrogen, gas[23] | 141.86 | 0.01005 | ||
| Diborane[24] | 78.2 | |||
| Beryllium | 67.6 | 125.1 | ||
| Lithium borohydride | 65.2 | 43.4 | ||
| Boron[25] | 58.9 | 137.8 | ||
| Methane (1.013 bar, 15 °C) | 55.6 | 0.0378 | ||
| Natural gas | 53.6[26] | 0.0364 | ||
| LNG (NG at −160 °C) | 53.6[26] | 22.2 | ||
| CNG (NG compressed to 250 bar/~3,600 psi) | 53.6[26] | 9 | ||
| LPG propane[4] | 49.6 | 25.3 | ||
| LPG butane[4] | 49.1 | 27.7 | ||
| Gasoline (petrol)[4] | 46.4 | 34.2 | ||
| Polypropylene plastic | 46.4[27] | 41.7 | ||
| Polyethylene plastic | 46.3[27] | 42.6 | ||
| Crude oil (according to the definition of ton of oil equivalent) | 46.3 | 37[26] | ||
| Residential heating oil[4] | 46.2 | 37.3 | ||
| Diesel fuel[4] | 45.6 | 38.6 | ||
| 100LL Avgas | 44.0[28] | 31.59 | ||
| Gasohol E10 (10% ethanol 90% gasoline by volume) | 43.54 | 33.18 | ||
| Lithium | 43.1 | 23.0 | ||
| Jet A aviation fuel[29]/kerosene | 42.8 | 33 | ||
| Biodiesel oil (vegetable oil) | 42.20 | 33 | ||
| DMF (2,5-dimethylfuran)[clarification needed] | 42[30] | 37.8 | ||
| Polystyrene plastic | 41.4[27] | 43.5 | ||
| Body fat metabolism | 38 | 35 | 22[31] | |
| Butanol | 36.6 | 29.2 | ||
| Gasohol E85 (85% ethanol 15% gasoline by volume) | 33.1 | 25.65[citation needed] | ||
| Graphite | 32.7 | 72.9 | ||
| Coal, anthracite[32] | 26-33 | 34-43 | 36 | |
| Silicon[33] | 32.2 | 75.1 | ||
| Aluminum | 31.0 | 83.8 | ||
| Ethanol | 30 | 24 | ||
| Polyester plastic | 26.0[27] | 35.6 | ||
| Magnesium | 24.7 | 43.0 | ||
| Coal, bituminous[32] | 24-35 | 26-49 | ||
| PET plastic | 23.5 (impure)[34] | |||
| Methanol | 19.7 | 15.6 | ||
| Hydrazine (toxic) combusted to N2+H2O | 19.5 | 19.3 | ||
| Liquid ammonia (combusted to N2+H2O) | 18.6 | 11.5 | ||
| PVC plastic (improper combustion toxic)[clarification needed] | 18.0[27] | 25.2 | ||
| Wood[35] | 18.0 | |||
| Peat briquette[36] | 17.7 | |||
| Sugars, carbohydrates, and protein metabolism[citation needed] | 17 | 26.2 (dextrose) | 22[37] | |
| Calcium[citation needed] | 15.9 | 24.6 | ||
| Glucose | 15.55 | 23.9 | ||
| Dry cow dung and cameldung | 15.5[38] | |||
| Coal, lignite[citation needed] | 10-20 | |||
| Sodium (burned to wet sodium hydroxide) | 13.3 | 12.8 | ||
| Sod peat | 12.8 | |||
| Nitromethane | 11.3 | |||
| Sulfur (burned to sulfur dioxide)[39] | 9.23 | 19.11 | ||
| Sodium (burned to dry sodium oxide) | 9.1 | 8.8 | ||
| Battery, lithium-air rechargeable | 9.0[40] | |||
| Household waste | 8.0[41] | |||
| Zinc | 5.3 | 38.0 | ||
| Iron (burned to iron(III) oxide) | 5.2 | 40.68 | ||
| Teflon plastic (combustion toxic, but flame retardant) | 5.1 | 11.2 | ||
| Iron (burned to iron(II) oxide) | 4.9 | 38.2 | ||
| ANFO | 3.7 | |||
| Battery, zinc-air[42] | 1.59 | 6.02 | ||
| Liquid nitrogen[clarification needed] | 0.77[43] | 0.62 | ||
| Compressed air at 300 bar (potential energy) | 0.5 | 0.2 | >50%[citation needed] | |
| Latent heat of fusion of ice[citation needed](thermal) | 0.335 | 0.335 | ||
| Water at 100 m dam height (potential energy) | 0.001 | 0.001 | 85-90%[citation needed] | |
| Storage type | Energy density by mass (MJ/kg) | Energy density by volume (MJ/L) | Peak recovery efficiency % | Practical recovery efficiency % |
Energy density of electric and magnetic fields[edit]
Electric and magnetic fields store energy. In a vacuum, the (volumetric) energy density (in SI units) is given by
where E is the electric field and B is the magnetic field. The solution will be in Joules per cubic metre. In the context of magnetohydrodynamics, the physics of conductive fluids, the magnetic energy density behaves like an additional pressure that adds to the gas pressure of a plasma.
In normal (linear and nondispersive) substances, the energy density (in SI units) is
where D is the electric displacement field and H is the magnetizing field.




No comments:
Post a Comment